Genomics
Genomics studies the structure, function, evolution and mapping of DNA and the genetic makeup of animals. It deals with the complete set of genes and genetic material found in a cell or organism.
Genomic technologies draw both producer interest and research investment in the beef industry. Seedstock selection is one common application, but genomics has found widespread adoption in forage and feed grain breeding, diagnostic tests, vaccine development, source attribution for food safety recalls and other uses.
On this page:
Key Points
|
Genetics 101
|
Definitions - Genetics: The study of individual genes and how they behave (heredity) - Genomics: The study of how genes interact with each other and their environment and how that influences phenotypes - Phenotype: the observable traits that result from an animal’s genotype interacting with the environment (ex. coat colour, feed efficiency, horned/polled etc.) |
DNA is the genetic code that determines how an organism grows, what it looks like, and how it performs in a specific environment. Found in all living things, DNA gets passed from one generation to the next, allowing these organisms to maintain or improve their ability to survive and thrive.
 DNA is a long chain, with each link of the chain containing a pair of four small molecules, known as base pairs. These molecules are abbreviated by the letters A, T, G, and C. This long chain is then coiled tightly into chromosomes. All cells in an organism contain a complete copy of that organism’s full genetic code.
DNA is a long chain, with each link of the chain containing a pair of four small molecules, known as base pairs. These molecules are abbreviated by the letters A, T, G, and C. This long chain is then coiled tightly into chromosomes. All cells in an organism contain a complete copy of that organism’s full genetic code.
Each cell has specialized machinery that reads the DNA code three letters at a time. These three-letter codes instruct the cellular machinery to start “reading” at a specific point. From that point, sets of three base pairs code for specific amino acids, then finally a three-letter code instructs the cellular machinery to stop reading. This segment - from start to stop - is referred to as a gene.
When the amino acids that are coded for by genes are linked together, proteins are created. Some genes code for specific proteins in the body, such as hormones, enzymes, plant proteins and muscle proteins. Other genes influence when specific genes are turned on or off, or how actively genes are expressed.
Sometimes when DNA is copied, small copying mistakes occur. A “T” may be accidentally replaced with an A, G, or C. These are called single nucleotide polymorphisms (SNPs), and are regularly referred to as “markers” or “SNP markers.” So far, beef geneticists have discovered around 30 million different SNPs. These SNPs largely dictate the physical differences we see among animals, including liveweight, stature, coat color, horns, marbling, or average daily gain, among others. Identifying all 35 million of these SNPs in a single animal would be very expensive, therefore labs make smaller “panels” of these SNPs, which makes genotyping more affordable to use in animal breeding. One can categorize these panels into five different categories based on SNP density, with panels of higher density costing more:
-
- Small panels: analyze just a few SNPs to around 2,000 SNPs. Generally used for parentage, genetic abnormality testing, coat colour, horns, etc.,
- Low Density (LD) panels: contain between 5,000 to 30,000 SNPs. This type of panel is used for genomic prediction/selection purposes when there are a lot of higher density genotypes available to refer to.
- Medium Density (50K) panels: have between 50,000 and 150,000 SNPs. This density is fairly common in beef genomic selection programs. Usually a population will begin by gathering a reference set of 50,000 SNP genotypes on influential animals in the breed, which are then used to predict what less dense genotypes mean for performance traits.
- High Density (HD) panels: analyze 500,000 to 1 million SNPs. This panel is less popular in beef as It comes at a high cost.
- Whole Genome Sequencing (WGS): occurs when an animal’s entire genome is sequenced (i.e. all 3 billion base pairs are read). This type of test is presently used primarily for research and is too expensive to be employed on farm.
Even though an animal’s genetics are determined at conception, many environmental factors can modify how genes are expressed. For example, an animal may have a very high genetic potential for weight gain, but if there is a drought or feed is limited, the animal will obviously be unable to fulfill that genetic potential. Some traits follow a simple inheritance pattern, meaning the presence of one gene will dictate whether or not an animal has a trait. Other traits require a combination of a few genes to see an effect, and even more have a more complex inheritance pattern in which multiple genes as well as the environment all interact to produce a phenotype.
Click below to download our genomics fact sheet:
The use of Genomic Tests in Cattle
There are a number of ways that DNA testing can be used on an operation, including as an aid in bull and replacement selection, breeding choices, sorting animals into management groups, pedigree verification, eliminating or reducing undesirable genetic conditions, or marketing. Implementing DNA tests on an operation can be very valuable, but it is important to have a defined goal in mind before investing in testing. The value of DNA testing to an operation is dependant on breed, number of animals tested, whether cattle are purebred or commercial, breeding goals, marketing scheme, as well as what records are being kept on farm.
For example, if you sell your calves at weaning and are looking to DNA parentage test your calves to determine which sires are most economically valuable (or sire the most calves with high birthweights) but are not recording individual calf weaning weights the test would be of limited value.

DNA samples for most genetic tests can be obtained through hair, skin tissue, blood, or semen. Sampling kits can be obtained from laboratories or third-party providers (e.g. breed associations) and will provide details on collecting the sample and what information needs to accompany the sample depending on the specific test ordered.
Genomically-Enhanced EPDs
Expected progeny differences (EPDs) are a prediction of the average differences in genetic merit that are expected to be passed on to the offspring. A full description of EPDs and how they can be used to make selection decisions on your herd can be found here. When looking at EPDs, it is important to note the accuracy. In young animals, EPDs are relatively inaccurate as they have not produced any progeny. As more progeny are produced and more information on offspring performance is available, the EPDs of the sire and dam become more accurate. Bull EPDs are usually more accurate than cow EPDs because the average bull has many more offspring than the average cow. Genomic tests can be used to increase accuracy of prediction without having to sire more calves. Genomically enhanced EPDs (GE-EPDs) can be produced for most breeds in Canada. This increases the accuracy of the EPD by the same amount as having added performance information from seven to twenty-three offspring, depending on the specific trait. This allows producers to better be able to better predict the genetic merit of progeny from young animals (heifers or yearling bulls) before they have produced any calves.
Adding genetic information to create an EPD does not guarantee that the EPD itself will be more desirable, just that it will be more accurate. For example, a bull’s actual calving ease EPD may improve, worsen, or stay the same after a GE-EPD evaluation. The major difference is that whatever that actual EPD number is, a producer can have more confidence that the bull’s calving ease will be reflected by the EPD due to the higher accuracy.
For someone looking to purchase bulls or replacement heifers, GE-EPDs should be used in the exact same way EPDs are used. The accuracy value for the specific trait will help you determine how much variation to expect.
DNA Parentage Testing
DNA parentage testing has been used in the purebred industry for many years and is now being adopted by commercial cow-calf producers as prices for the tests drop. Generally, a parentage panel contains 120 SNPs used to determine which bull sired which calf. The present cost of parentage using a 120 SNP parentage panel is $12-$20.
Producers might adopt DNA parentage testing on their operations for several reasons. They may need to verify the sire for purebred registry requirements or for parentage discovery if cows are bred in multi-sire pastures. Producers may want to identify a ‘problem’ bull on their operation, such as a bull that sires high birth weight calves, low weaning weight calves, or fewer calves than expected. If the right records are kept on farm, parentage testing can help identify superior sires (e.g. bulls siring high weaning weight calves or calves with a high ADG), identify sires that are producing calves with desired traits, or sires that are not breeding many cows.
Parentage verification SNP tests take advantage of the fact that half a calf’s DNA is inherited from the sire and half from the dam. The tests look at specific SNPs from both the calf and all possible sires. A process of elimination then determines the sire - if the calf carries a SNP not carried by a particular bull, that bull can be excluded as the sire. Therefore, it’s important to submit samples from all possible sires. Sometimes, especially in cases where potential sires are closely related, DNA from only the sire and calf is not enough to narrow down which bull sired the calf and a sample from the dam may be required.
Trait Assessment
An animal that has inherited the same version of a particular gene from each parent is called homozygous. If an animal receives a different form of the gene from each parent, it’s called heterozygous. Heterozygous animals can pass on either form of the gene to their offspring,
SNP tests can be used to determine if animals are “carriers” for a specific trait. Recessive traits require that the offspring inherit a copy of the gene from each parent before the recessive trait is expressed. Otherwise, the dominant copy from one parent (e.g. polled) will mask the recessive copy from the other parent (e.g. horned). SNP tests can help to unmask carriers for some traits.
One example of a simply inherited dominant/recessive trait is coat colour. For example, in black cattle, red hair is the recessive form of the gene, so even if an animal is black, it may still carry a red gene, and could pass the red gene to its calves. If two carriers are bred to each other, there is a one-in-four chance their offspring will express the recessive trait even though neither of the heterozygous parents do. Following is an example showing what happens when mating a cow and bull that are both heterozygous black.
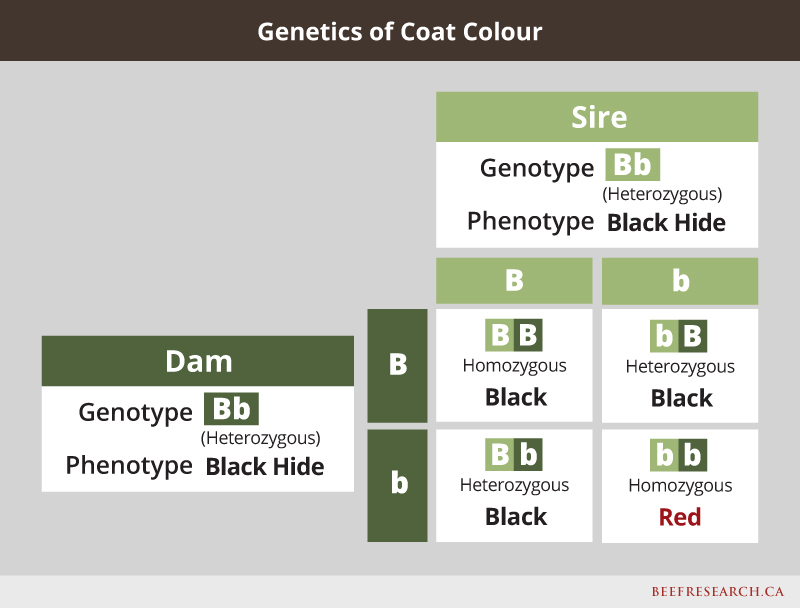
Past research funded by the Canadian Beef Cattle Check-off validated that a mutation in the calpastatin gene was associated with differences in beef tenderness and is controlled by this simple inheritance pattern.
Genetic Abnormality Testing
Abnormalities in the skeleton, body formation, or the way the body functions occasionally occur in beef cattle. They can be caused by the environment (e.g. crooked calf disease caused by lupine consumption), genetics, or both. If environment plays a factor, adjustments can be made to prevent the disease in the future. If the disease is genetic, testing may be required to correct or manage the problem.
Some examples of genetic abnormalities are: Chondrodysplasia (dwarfism), Hypotrichosis (hairlessness), Arthrogryposis Multiplex (Curly Calf Syndrome) and Syndactyly (mulefoot).
Genetic abnormalities are often breed specific, only occur in a few breeds, or only occur in specific bloodlines within a breed. Some breed associations are working to reduce the frequency of genetic defects within their breeds by using genetic testing to identify carriers. Most genetic abnormalities follow a simple dominant/recessive inheritance pattern (like polled and horned in the example above), so you can avoid genetic abnormalities by avoiding mating carriers to each other.
Genetic abnormalities can result in abortion, or death shortly after birth. If you suspect that you are seeing a genetic abnormality in your herd, talk to your veterinarian to determine what’s causing the defect. If it’s genetic, test your bulls for carrier status. Avoid buying bulls that are carriers for the defect, or crossbreed to a different breed that doesn’t have the abnormality.
Genetic Tests to Sort Animals into Management Groups
 Genetic tests can be used to manage groups of cattle. Tests can give producers a better idea of how animals will perform in specific situations. These tests enable producers to sort animals into particular management groups. This is commonly referred to as marker assisted management (MAM).
Genetic tests can be used to manage groups of cattle. Tests can give producers a better idea of how animals will perform in specific situations. These tests enable producers to sort animals into particular management groups. This is commonly referred to as marker assisted management (MAM).
For example, the leptin gene codes for the hormone leptin that regulates appetite and fat deposition. For this particular gene, cattle will have a base pair code of CC, TC or TT. The TT calves will typically deposit backfat faster and require fewer days on feed than TC or CC calves. By leptin testing, feedlot operators can sort calves into more uniform groups to feed and sell. Recently a genetic test has been developed that allows producers to know the breed composition (or heterozygosity) of an individual animal, which can help producers with future breeding decisions if they wish to maximize heterosis.
Genomics works very well for parentage testing, controlling inbreeding, and testing for many genetic abnormalities. However, caution must be exercised when using genomics for selection or management. When traits are controlled by a single gene, selection is relatively simple. That being said, even though some traits have reliable and accurate tests, single trait selection, for any one trait is not advised as selecting for only one trait often means selecting for or against other traits unintentionally. Most production traits (e.g. growth or fertility) are controlled by hundreds of different genes that all interact with each other, which makes those traits challenging selection targets. For example, calves from a homozygous polled dam will always be polled because they are guaranteed to inherit at least one copy of the polled gene. But, if a dam had a short calving interval every year, it is not guaranteed that her progeny will display that same short calving interval because there are several different genes that influence calving interval and it is unlikely her calves will inherit all the copies that produce the most desirable result.
While genetic testing works very reliably in cases where the SNP is known to occur within an actual gene (i.e. functional or causative mutation), in many cases the SNP may only be located somewhere near the gene. In those instances, the accuracy of GE-EPDs will depend on the relationship between the animals tested and the population from which the prediction equations used to generate the GE-EPDs were developed. GE-EPD prediction equations using SNPs that were discovered in one bloodline may not work as reliably in another, and GE-EPD prediction equations developed using SNPs that were discovered in one breed are unlikely to produce reliable results in a different breed. Since the beef cattle herd primarily consists of crossbred animals, the inability to provide SNP markers that reliably predict crossbred animal performance has been a major barrier to the adoption of genomic selection in the commercial cattle population.
Diagnostic Tests
Numerous diagnostic tests use genomics. Advancements in genomics have improved the ability to detect and understand many animal diseases.
Because researchers can now map the genome of a specific virus or bacteria, detecting them in a host animal becomes easier. For example, genomics helped develop improved tests for trichomoniasis (trich) and bovine genital campylobacteriosis (vibrio). The trich test can detect pathogenic trichomonads and ignore harmless trichomonads that may also be present, while the vibrio test improved upon traditional culture methods that often resulted in false negatives. Using genomic technologies, more rapid, accurate and cost-effective diagnostic tests are now available.
Researchers also use genomic technologies to improve current diagnostic tests. Research funded through the BCRC is developing a single, rapid, cost-effective test that can detect a range of respiratory and enteric diseases.
Disease Outbreak and Food Safety Investigations
In the case of disease outbreaks, genomics and genetic testing of the causative agent can play a major role. Scientists are able to take a sample from an infected animal and map the genome of the causative bacteria. By examining the genetic sequence of the pathogen, it may be possible to determine the origin of the infection, if multiple strains are involved (suggesting multiple routes of infection or transmission), and can help to inform control strategies or policy.
Genomics has made major advancements in the area of traceability. In the event of a food safety recall, genetic tests can be used to link bacterial DNA to the processing facility and identify where along the production chain the contamination occurred.
Compared to bacterial culture, genomic testing increases both the accuracy and the speed with which food safety outbreaks can be traced.
Vaccine Development
In the same way genomics plays a role in looking at the genome of pathogens in outbreak investigations it also works in developing more effective vaccines, discovering new infectious agents, and providing a better understanding of known vaccines and pathogens.
Vaccines encourage a cow’s immune system to develop antibodies against specific disease organisms. An important part of vaccine development is finding a unique protein on the bacteria or virus (antigens) that the animal’s immune system recognizes and then stimulates the immune response.
Genomics can aid in identifying the best proteins to stimulate immunity and makes it much easier to multiply and produce these proteins in large quantities. This increases the effectiveness of the vaccine by making it more specific, with fewer side effects. It also decreases the cost of producing the vaccine.
 Plant breeders have been using genomics for years to help select valuable traits. Understanding a plant’s genetic sequence allows plant breeders to combine genomic tools with traditional breeding methods. That helps shorten the time it takes to develop new varieties and makes it easier to select for complex traits.
Plant breeders have been using genomics for years to help select valuable traits. Understanding a plant’s genetic sequence allows plant breeders to combine genomic tools with traditional breeding methods. That helps shorten the time it takes to develop new varieties and makes it easier to select for complex traits.
The use of genomic technology allows plant breeders to develop varieties with characteristics that are more robust, disease resistant, higher yielding, cold tolerant, winter hardy and so forth. Genomics allows plant breeders to identify superior lines more quickly and accurately, and make more strategic selection and breeding decisions, resulting in more rapid genetic progress.
Current research funded by the Canadian Beef Cattle Check-off is using genomics as a tool to develop improved tame and native forages.
Future Potential
Our understanding of genomics continues to evolve. Ongoing research on the genetic improvement of beef cattle utilizing genomic testing includes whether we can select for improved disease resistance or maternal production traits to inform replacement heifer selection. These areas continue to evolve, but other areas related to the beef industry continue to advance at an even more rapid pace. Genetic traceability is now possible and some value chains are marketing full farm to plate traceability through DNA sampling. New genomic methods of plant breeding are being used and developed, and any future chute side disease diagnostic platforms will likely incorporate genomics. As our understanding of genomics and genetics grows, the potential to use these technologies to benefit multiple aspects of beef production continues to expand.
Feedback
Feedback and questions on the content of this page are welcome. Please e-mail us.
This topic was last revised on October 9, 2020 at 11:09 AM.



 View Web Page
View Web Page View PDF
View PDF


