Antibiotic Resistance
Antimicrobial resistance occurs naturally when the genetic make-up of microbes is altered in a manner that makes them no longer susceptible to antimicrobials designed to kill them or prevent their growth. In Canada, surveillance indicates that resistance levels in cattle and beef are extremely low and have not increased over time. Research and surveillance evidence suggest that eliminating antimicrobial use in beef production would have clear negative health consequences for cattle with no obvious benefit for human health. See introduction section below for definitions of 'antimicrobial' and 'antibiotic'.
On this page:
- Key Points
- Introduction / Video
- Function of Antimicrobials
- Mutation of Microbes
- Categories of Antimicrobials
- Concerns in Cattle Production
- Concerns in Public Health
- Surveillance of Antimicrobial Resistance in Beef Cattle
- Misconceptions in Antimicrobial Use and Resistance in Livestock
- Prescription only
- Antimicrobials in cattle feed
- Using Antimicrobials to control disease
- Using antibiotics on animals with viral infections
- Monitoring the amount administered
- Avoiding Antimicrobial Resistance
- Using Antimicrobials Responsibly in Beef Cattle
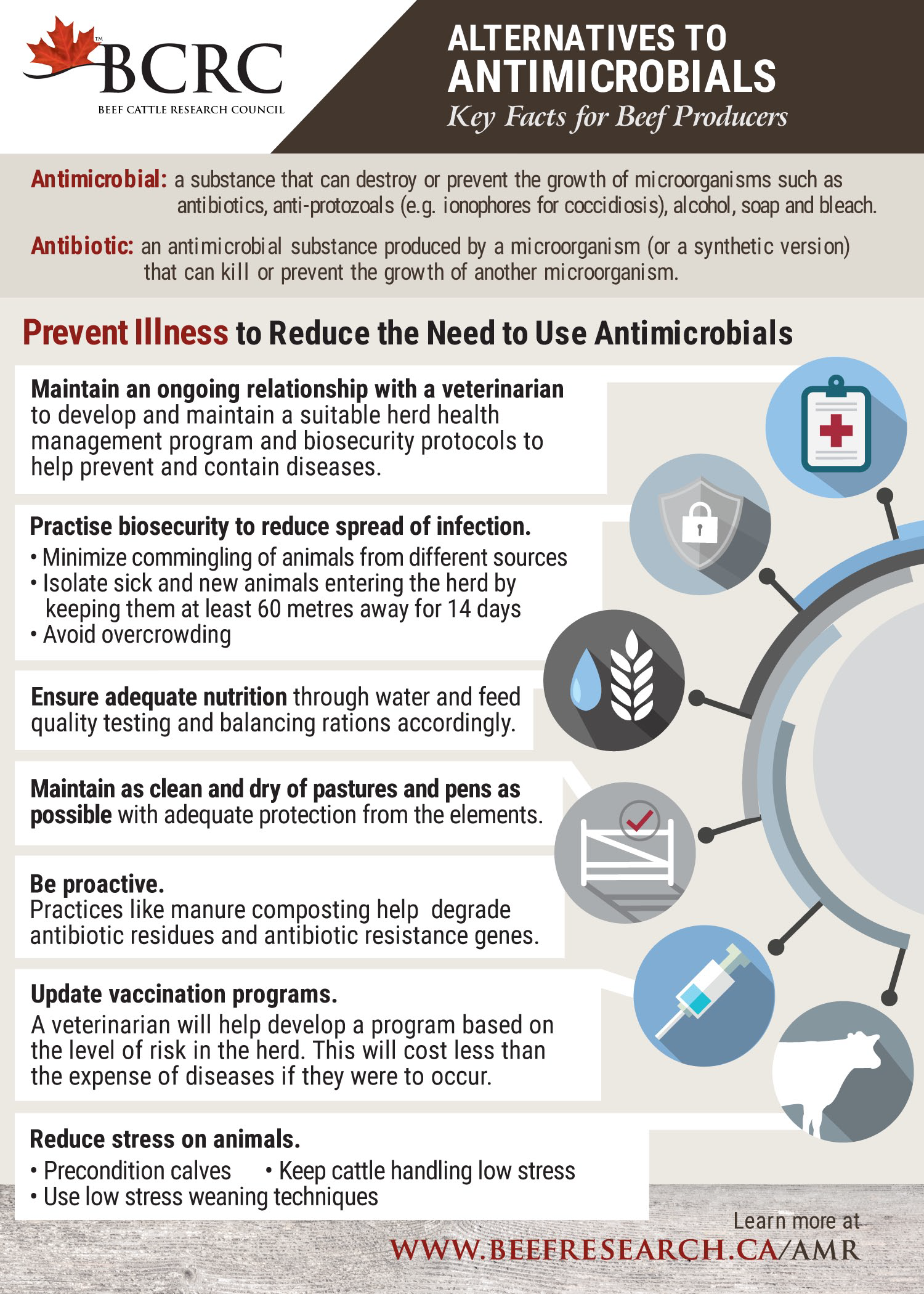
- Preventing Illness in Beef Cattle to Reduce the Need to Use Antimicrobials
- National Beef Antimicrobial Research Strategy
Key Points
|
Introduction
Antimicrobial: a substance that can destroy or prevent the growth of microorganisms. There are many different types of antimicrobial substances, including antibiotics, anti-protozoals (e.g. ionophores for coccidiosis), biocides like alcohol, soap and bleach.
Antibiotic: an antimicrobial substance produced by a microorganism (or a synthetic version) that can kill or prevent the growth of another microorganism. In human and veterinary medicine, antibiotics are used to treat bacterial infections.
All antibiotics are antimicrobials, but not all antimicrobials are antibiotics. The two terms are often used interchangeably.
Medically Important Antimicrobial: Antimicrobials considered to be important for the treatment of bacterial infections in humans.
Some drugs are considered more important than others in the treatment of serious bacterial infections, and resistance development against those antimicrobials might have more serious consequences for human health.
Antimicrobials are used in aquaculture, fruit production, beekeeping, livestock production, pets, wildlife, some crops, industrial and household chemicals and human medicine. In livestock, various categories of Medically Important Antimicrobials are used for therapy (i.e. treat illness) or disease control. The use of Medically Important Antimicrobials for production purposes in livestock (i.e. improve feed efficiency) has been banned in Canada. Other management tools used to prevent and control disease in beef production include nutrition, hygiene, vaccines, housing, anti-parasitics. probiotics and enzymes.
Function of Antimicrobials
Nearly all antimicrobials are derived from those naturally produced by soil microorganisms. Imagine that two types of microbes share the same environment and rely on the same nutrient source to exist. Microbe A will have a competitive advantage if it can produce and excrete an antimicrobial that inhibits or kills microbe B, and vice-versa. At the same time, microbe A may have naturally evolved a resistance mechanism to protect itself against its own antimicrobial so that it doesn’t accidentally kill itself when it produces it – this is called “intrinsic resistance”.
Antimicrobials are used in human and veterinary medicine for the same reasons. Using an antimicrobial to target specific microbial pathogens is an effective way to combat infectious disease, especially when effective vaccines are not available.
Antimicrobials harm or kill their target microbe by damaging or blocking specific structural features (e.g. the cell wall or a cell surface receptor) or metabolic functions. Sometimes target microbes undergo random genetic mutations or acquire resistance genes from another (resistant) microbe. This can change structural features or metabolic processes that make the target microbe and its offspring resistant to that antimicrobial (or related antimicrobials that kill or prevent growth in the same manner).
Mutation of Microbes
| It is important to recognize that mutation is not caused by the presence of an antimicrobial |
Antimicrobial resistance occurs naturally and is not caused by antimicrobial use. If the antimicrobial is used when an antimicrobial resistant disease-causing microbe (pathogen) is present, the antimicrobial resistant pathogen will have a competitive advantage over its susceptible cousins.
If the antimicrobial continues to be used, the resistant pathogens will survive, reproduce and become more common, while the susceptible pathogens will gradually become more scarce. In this case, the antimicrobial will become less effective, and the animal will not respond to continued treatment with the same antimicrobial, even if the dose is increased. In this case, the veterinarian or doctor may switch to an antimicrobial from a different class or category.
Categories of Antimicrobials
Antimicrobials are divided into four categories based on their importance in human medicine. Each category of importance contains antimicrobial drugs of different classes. Classes of drugs are based on their chemical makeup.
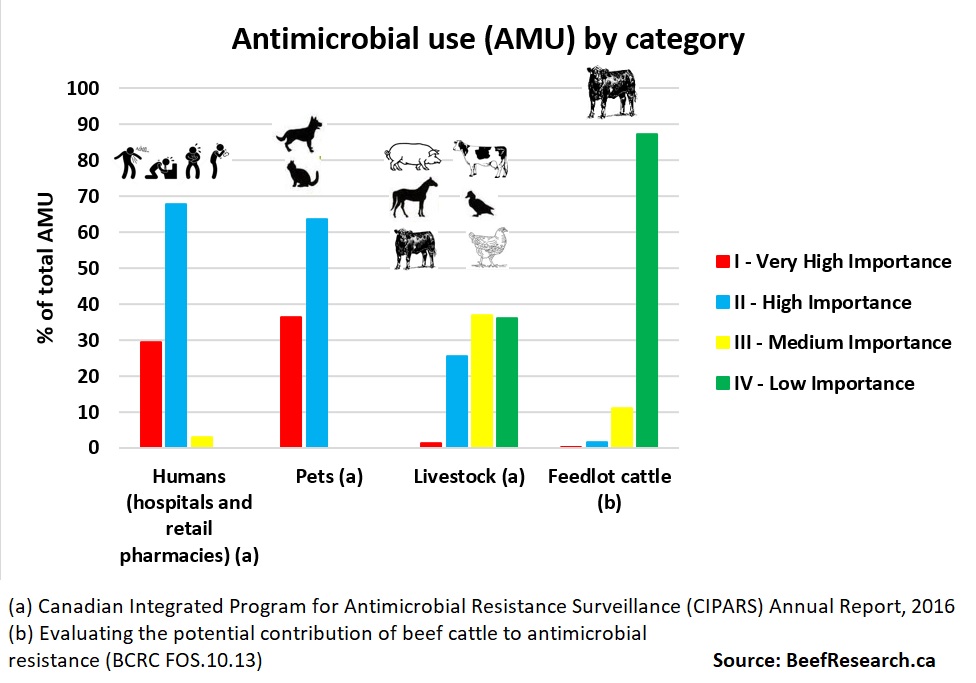 Antimicrobials classified as ‘Very High Importance’ are used to treat very serious human infections.
Antimicrobials classified as ‘Very High Importance’ are used to treat very serious human infections.- ‘High Importance’ antimicrobials are of intermediate concern in human medicine.
- ‘Medium Importance’ drugs are rarely used to treat serious human health issues. For example, tetracycline used to treat acne is classified as Medium Importance.
- Antimicrobials of ‘Low Importance’, like ionophores, are not used in human medicine to treat bacterial infections.
In Canada, Medically Important Antimicrobials include those in the Very High, High and Medium Importance categories. Low Importance Antimicrobials are not classified as Medically Important. Antimicrobials from all four categories (Low, Medium, High or Very High importance) are registered for use in beef cattle in Canada. The majority of antimicrobial doses used in Canadian beef production are of Low (category IV) importance in human health. The majority of Medically Important Antimicrobial doses used in Canadian beef cattle are of Medium (category III) importance.
Antimicrobials of Low importance include ionophores, which are used in beef cattle to prevent diseases such as coccidiosis and to improve feed efficiency. Generally category II and III antimicrobials are used for treatment or control of bacterial infections. In Canada, Category I antimicrobials (Very High importance) are seldom used in beef cattle production and only for treatment (not control or prevention) of severe bacterial infections in overtly sick animals.
| Category of Importance in Human Medicine |
Antimicrobial Class | Brand Names of Antimicrobial Products Registered for Use in Beef Cattle in Canada |
| I: Very High | e.g. fluoroquinolones | e.g. Baytil, A180 |
| e.g. 3rd/4th generation cephalosporins | e.g. Excenel, Excede | |
| II: High | e.g. macrolides | e.g. Tylan, Micotil, Draxxin, Zuprevo, Zactran |
| III: Medium |
e.g. tetracyclines e.g. phenicols |
e.g. Liquamycin, Aureomycin e.g. Nuflor, Resflor |
| IV: Low | e.g. ionophores | e.g. Rumensin, Bovatec, Posistec |
Concerns in Cattle Production
Antimicrobial resistance is a concern in livestock production for two reasons:
- If pathogens develop resistance, the antimicrobials will stop working, animals will not respond to treatment, and the risk of animal health and welfare concerns and death may increase.
- Antimicrobial resistant livestock pathogens may be able to pass their resistance on to human pathogens. This would result in antimicrobial drugs not being as effective in treating human infections as well.
Concerns in Public Health
| The use of all antimicrobials in veterinary medicine are approved and regulated by Health Canada. |
The greatest concern is with antimicrobials that are of Very High Importance in human medicine, but that are also used in livestock. These are drugs of last resort in human medicine (and in veterinary medicine); they are used for infections that are not responding to lesser drugs, and for which there are no other alternatives. If the Very High Importance drugs fail to work, doctors (and veterinarians) have no other options.
| Producers work with their veterinarians to develop protocols on when it's appropriate to use various antimicrobials, and adhere to withdrawal times to ensure treated animals do not enter the food system until it's safe to do so. |
Ionophores (classified as 'Low Importance' in human medicine) are often erroneously included in discussions about the concern of antimicrobial use in livestock and the potential link to antimicrobial resistance in humans. Ionophores are not used in human medicine to treat infections and have a very different mode of action than other antibiotics. There is no evidence that ionophores lead to cross-resistance to antibiotics of importance in human medicine.
The potential link between antimicrobial use in cattle production and antimicrobial resistance in human medicine has been very difficult to prove or disprove. One study funded by the Canada-Alberta Beef Industry Development Fund found that bacteria isolated from feedlot staff who worked both with sick cattle and the drugs themselves had antimicrobial resistance levels that were no higher (and were lower in many cases) than in bacteria collected from human health labs.
The Canadian beef industry has supported a number of research projects studying this issue since the late 1990’s. Two studies funded through the Beef Cattle Research Council (BCRC) have found that drugs that are most important for human health are rarely used by the Canadian beef industry. Conversely, the Low Importance antimicrobials that are used most widely by the Canadian beef industry are never used to treat bacterial infections in human medicine.
Most recently, research supported through the Beef Science Cluster has examined the risk that antimicrobial residues, resistant bacteria or resistance genes can travel from feedlot environments to human environments, through manure, soil, and water. This study found that composting manure is an effective way to dissipate antimicrobial residues and resistance genes. Furthermore, wetlands and microbiologically active soils play an important role in degrading antimicrobials and resistance genes. Consequently, microbial populations and antimicrobial resistance profiles from feedlot and human associated environments differ significantly.
Bacteria that have adapted to thrive in one particular environment won’t be able to compete with their close relatives who have adapted to thrive in a different environment. For instance, Enterococci are a group of bacteria that are commonly found in both cattle and humans. But there are many, many different kinds of Enterococci, each with their own preferred environmental niche(s). Enterococcus hirae are the most common Enterococcus in beef cattle (and rarely found in humans), while Enterococcus faecium and Enterococcus faecalis are commonly found in humans, but are rarely found in cattle. Enterococcus faecium and E. faecalis are the enterococci that most often cause infections in humans.
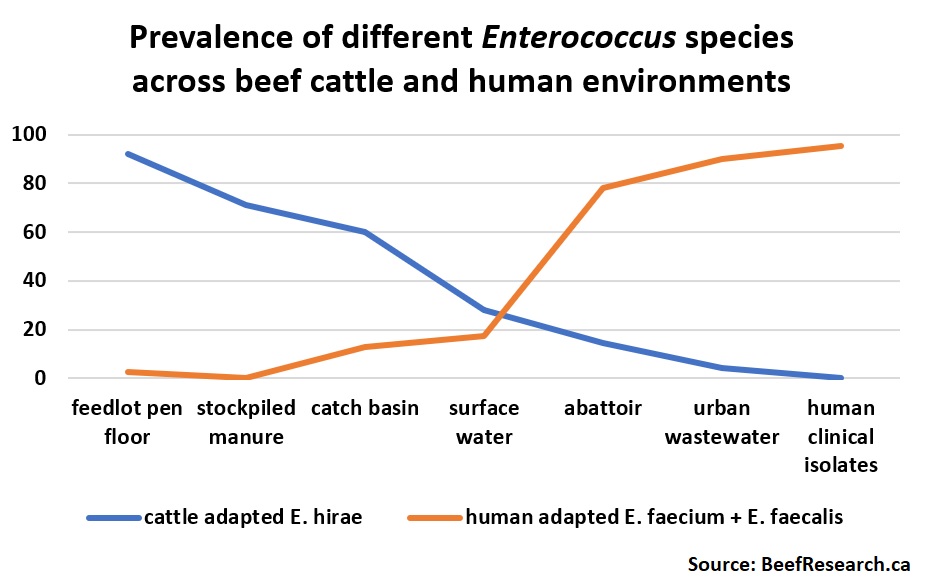
Even when E. faecium and E. faecalis were occasionally found in cattle, their genetic makeup and antimicrobial resistance patterns differed from the E. faecium and E. faecalis found in human patients. The E. faecium and E. faecalis occasionally found in cattle also lacked the genes required to induce illness in people. This strongly suggests that antimicrobial use practices in beef production influence antimicrobial resistance patterns in cattle-associated environments, while antimicrobial use practices in human medicine influence antimicrobial resistance patterns in humans.
One feedlot in the study raised some pens of cattle using antibiotics (“conventional production”) as well as not using antibiotics in other pens of cattle (“natural production”). The same types of antibiotic resistance genes were found in both conventional and natural pens. However, a larger total number of antibiotic resistance genes were found in the conventional pens. This underscores the fact that the more antibiotics are used, the more common antibiotic resistant bacteria will be found in cattle. This means that responsible antibiotic use in cattle is critically important to make sure they continue to be effective in treating cattle illness, even if it doesn’t pose a downstream risk to human health. It also proves that not using antibiotics does not result in all bacteria losing their antimicrobial resistant genes, again emphasizing that antibiotic resistance is a natural phenomenon.
Surveillance of Antimicrobial Resistance in Beef Cattle
The Public Health Agency of Canada has developed the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) to monitor antimicrobial resistance in humans and livestock, and retail meat. Surveillance results fluctuate from year to year, but CIPARS results to date indicate that antimicrobial resistance is relatively low. Multidrug resistance is similarly low and is not increasing.
This low level of resistance is probably because drugs of Very High Importance are very rarely used in beef cattle in Canada; drugs of last resort in human medicine are also drugs of last resort in bovine medicine.
| Research and surveillance evidence suggests that eliminating antimicrobial use in beef production will have clear negative health consequences for cattle with no obvious benefit for human health. |
Building on antimicrobial use and resistance surveillance frameworks developed in collaboration with the beef industry, CIPARS has implemented an Alberta feedlot component to its on-farm surveillance programs in 2016. In 2019 this pilot was expanded to include more feedlots in Alberta, as well as lots in Saskatchewan and Ontario.
While much of the antimicrobial use and resistance research in beef cattle has focused on the feedlot sector, antimicrobial use has also been studied in Canada’s cow-calf sector. A survey of 100 cow-calf operations enrolled in the Western Canadian Cow-Calf Surveillance Network found that while Medically Important Antibiotics were used nearly all herds, most were category III (Medium Importance) antimicrobials, and were used in fewer than 5% of cows, calves or bulls in most herds. Lower antimicrobial use in cow-calf herds (compared to feedlots) likely results from a lower disease challenge related to lower stress, less co-mingling of animals from multiple sources, and a lower stocking density on pasture compared to confinement pens.
Misconceptions in Antimicrobial Use and Resistance in Livestock
Livestock industries are also often criticized due to misinformation and misunderstanding around the availability and use of antimicrobials in livestock production.
Prescription Only
| In simple terms, A VCPR means that your veterinarian understands your operation, your management practices, your herd, and common health issues well enough to provide meaningful advice and oversight |
In Canada, Medically Important Antimicrobials for cattle are only available by veterinary prescription, within the confines of a valid veterinary-client-patient relationship (VCPR)
Cattle producers cannot legally purchase medically important antimicrobials over-the-counter without a prescription in Canada.
Antimicrobials in cattle feed
Medically important antimicrobials cannot be labelled or used for growth promotion of cattle in Canada. All in-feed medically important antimicrobials require a veterinary prescription before the feed can be formulated and/or sold.
In some cases, the method of drug delivery is confused with why it is being used. For example, when a drug is fed to an animal, rather than injected, an incorrect assumption may be made that the purpose of the fed medication is to promote growth. In fact, if a large number of animals need to be treated on a daily basis for a period of time, it is much safer and less stressful for the animal to deliver the drug through feed or water than to handle each individual repeatedly for daily drug injections.
Water or feed may be used to deliver oxytetracycline and chlortetracycline to feeder calves at high risk of respiratory disease. Similarly, oral tetracycline may be prescribed to teenagers to treat acne, not to make them grow faster.
A poor appetite is a common sign of respiratory disease in cattle.Keeping animals healthy keeps them eating and growing, which may also contribute to the confusion between antimicrobial use for health purposes and growth promotion. However, giving antimicrobials to healthy cattle does not improve growth rate or feed efficiency.
Using antimicrobials to control disease
Metaphylaxis is the treatment of a group of animals to combat a disease outbreak or to control the spread of infection when the risk of developing disease is high. This strategy may be used to prevent respiratory disease in high-risk (lightweight, freshly-weaned, auction mart derived) calves. Newly arrived feedlot calves may be stressed, depending on whether they have been recently weaned, how far they have been transported, and whether they have been adapted to dry feed. Stress can impair immune function. Since effective vaccines are not available for all of the respiratory pathogens and because vaccines are not effective if they are administered after feedlot cattle have already started to show signs of illness, antimicrobials may be used to control the spread and development of disease for the first few weeks in the feedlot. Once the calves have adapted and overcome the stressors, the incidence of disease is very low.
Use of Medium or Low Importance antimicrobials to effectively control or prevent disease in cattle can reduce the need to use more powerful antimicrobial drugs of High or Very High Importance to treat and cure disease once the disease has progressed and become more serious.
Using antibiotics on animals with viral infections
| Viruses are not susceptible to antibiotics. |
An antibiotic will not be effective against calf scours caused by rotavirus or coronavirus, or respiratory diseases caused by viruses (e.g. BVD, IBR, PI-3, BRSV). However, these conditions may be treated with antibiotics to reduce the risk of secondary bacterial infections that can result in pneumonia.
Monitoring the amount administered
| Commercial and on-farm feed mills are under the regulation of the Canadian Food Inspection Agency |
Modern cattle feeding operations and feed mills are equipped with highly sophisticated feed processing, mixing and delivery equipment that help to ensure that each animal receives the amount of antimicrobial needed, if delivered through the feed. Injectable antibiotics are delivered even more precisely, with a specific volume given to an animal depending on its individual body weight.
| View the Global Roundtable for Sustainable Beef (GRSB) Statement on Antimicrobial Stewardship |
Producers who participate in Canada’s Verified Beef Production PlusTM (VBP+) program demonstrate that they follow industry-sanctioned practices which select, use, store and dispose of antimicrobials in a responsible manner.
National Beef Antimicrobial Research Strategy
The National Beef Antimicrobial Research Strategy was developed by the Beef Cattle Research Council (BCRC) and the National Beef Value Chain Roundtable (BVCRT) following comprehensive analysis of the antimicrobial research situation relevant to the Canadian beef sector, extensive consultation and validation with all major stakeholder groups, and collaboration with funders toward coordinating and aligning funding priorities. This strategy identifies priority research outcomes for the Canadian beef industry and has gained the commitment from Canada’s major research funders to focus on achieving these outcomes.
Research outcomes have been defined in the priority areas of:
- Antimicrobial Resistance,
- Antimicrobial Use, and
- Antimicrobial Alternatives
Feedback
Feedback and questions on the content of this page are welcome. Please e-mail us.
Acknowledgements
Thanks to Dr. Tim McAllister (Agriculture and Agri-Food Canada) and Dr. Cheryl Waldner (Western College of Veterinary Medicine) for contributing their time and expertise during the development of this page.
This topic was last revised on January 10, 2020 at 2:01 AM.





 View Web Page
View Web Page View PDF
View PDF


